
As p shell can allow six electrons, there is a scarcity of only two electrons.ĭue to this, the maximum valence electrons in a single oxygen atom is six. Now, for the oxygen atom, its atomic number is eight whereas the electronic configuration is 1s2 2s2 2p4. Let us move to hydrogen, where its atomic number is one, and electronic configuration is 1s1.Īs s shell can accommodate up to two electrons, there is a scarcity of only one electron, a hydrogen atom needs only one valence electron to complete its shell. So, carbon needs four electrons to complete its octet. As p shell can accommodate up to six electrons there remains a scarcity of four electrons. The atomic number of carbons is six where its electronic configuration is 1s2 2s2 2p2. Moreover, in the case of the lewis structure, the electrons are always drawn in the pairs, whereas, the unpaired electrons mostly predict the dearth of the valence electrons. So, all the calculations will be done keeping eight as the maximum number for one atom. It arises from the need for achieving an electronic configuration similar to that of the noble gases. As per the octet rule, the total number of electrons that an atom can accommodate is eight.Īs per rule, the total number of valence electrons an atom can accommodate in its outermost shell is eight.
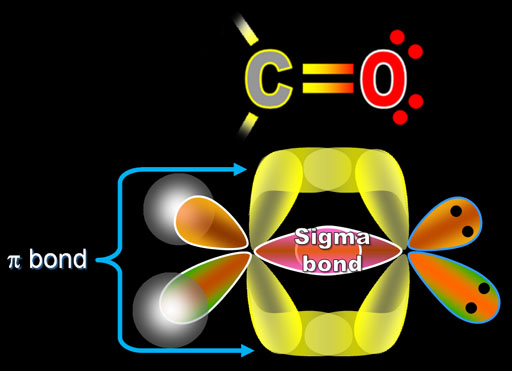
The valence electrons are present in the outer shell of an atom, which actively participates in the bond formation either by getting donated or accepted.Īs they are present in the outermost shell, the hold of the nucleus on the valence electrons in the outermost shell is weak.ĭue to this, the valence electrons can readily participate in the bond formation to stabilize their octet.
Conclusion What are the Valence electrons?


 0 kommentar(er)
0 kommentar(er)
